Chronic Obstructive Pulmonary Disorder (COPD) Inhaler Criteria
The following information has been copied from the BC Government Website.
Click here for the entire update : Link to BC Government PharmaCare Coverage
“Effective July 7, 2020, PharmaCare has updated the coverage of inhalers for the treatment of chronic obstructive pulmonary disease (COPD).
The updates align the therapeutic approach for COPD with current best clinical practices, improve patient outcomes and safety, and ensure the appropriate use of health care resources.
The updates will
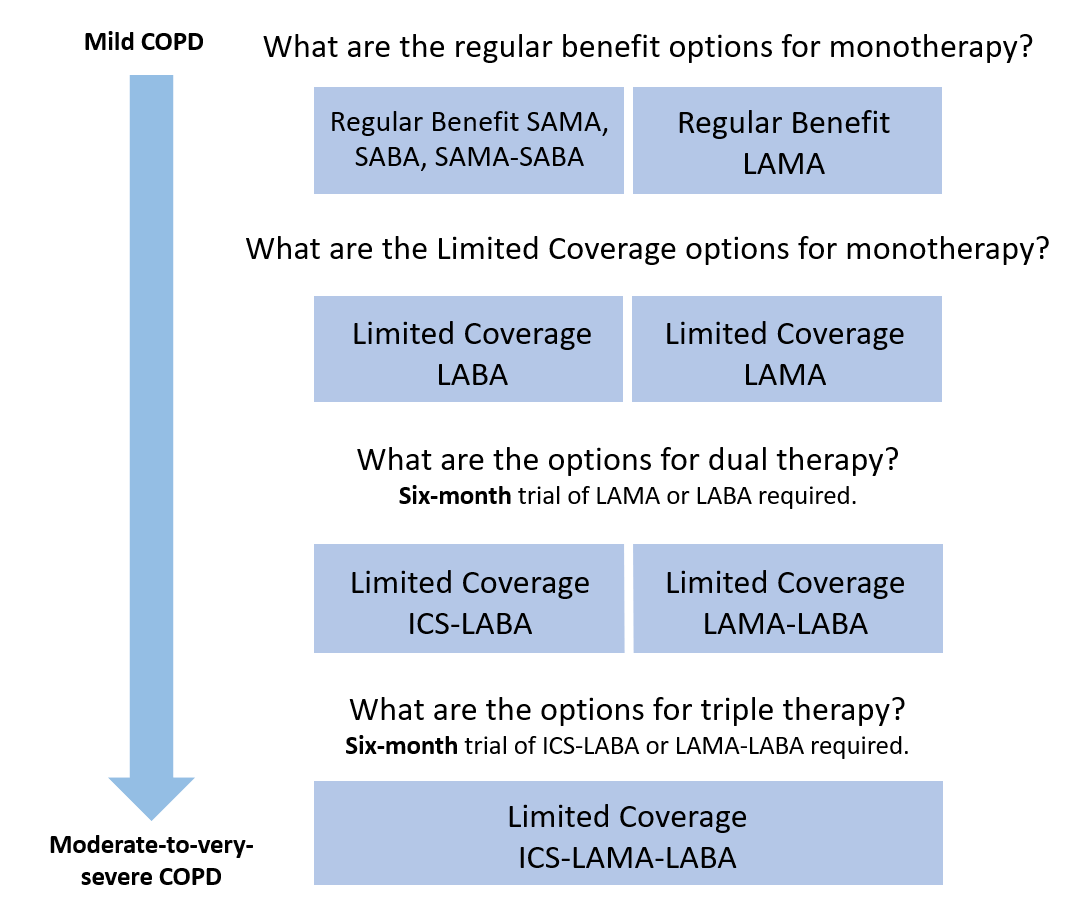
- Improve access to long-acting muscarinic antagonists (LAMA) by making tiotropium (Spiriva® Respimat®) and umeclidinium (Incruse® Ellipta®) regular benefits
- Modify the Limited Coverage criteria for other LAMAs, LABAs, LAMA-LABAs and ICS-LABAs
- List fluticasone-umeclidinium-vilanterol (Trelegy® Ellipta®) as a Limited Coverage benefit
The following is a stepwise approach to PharmaCare coverage of COPD inhalers: